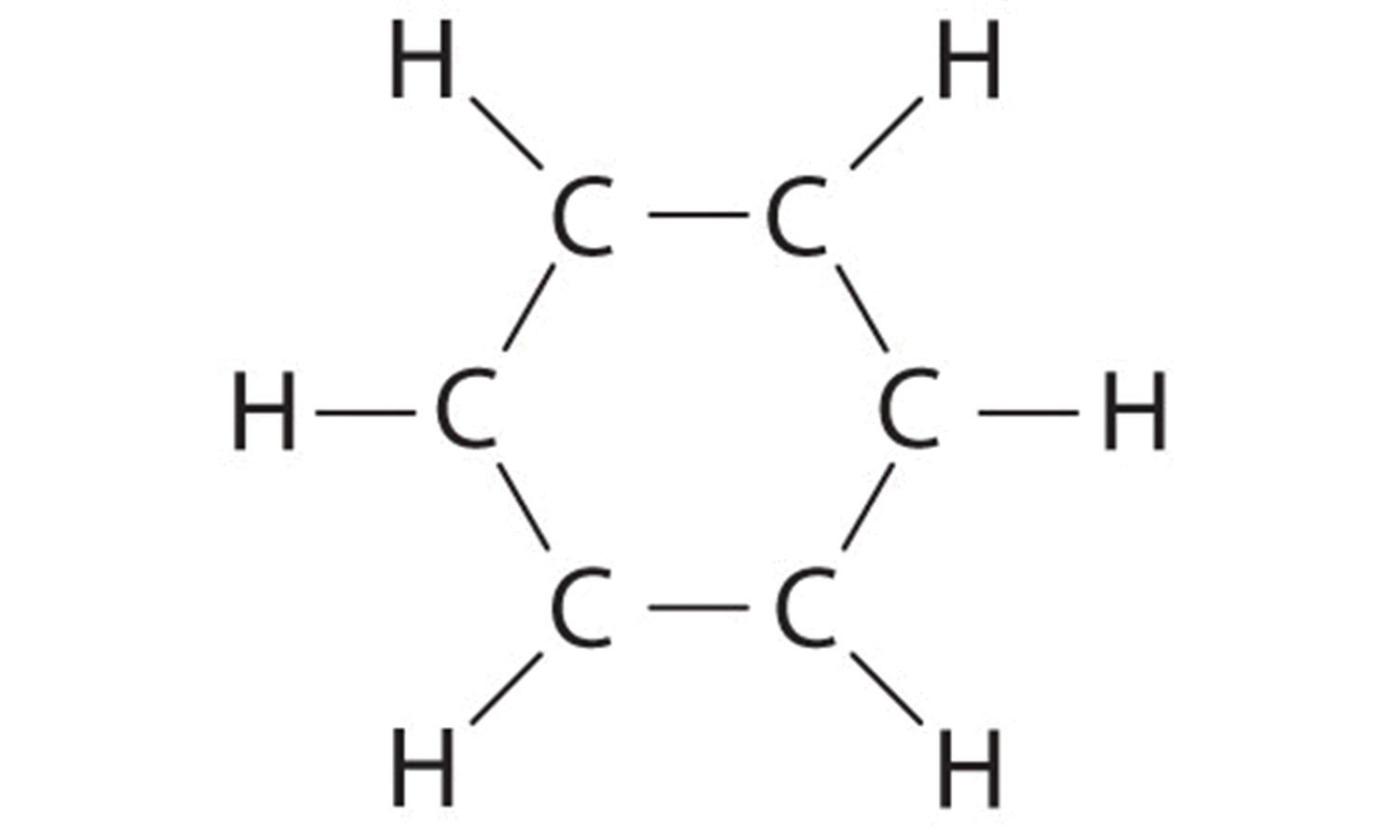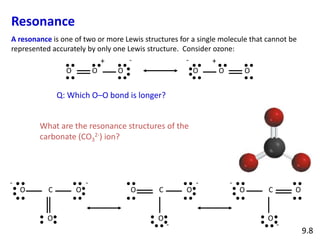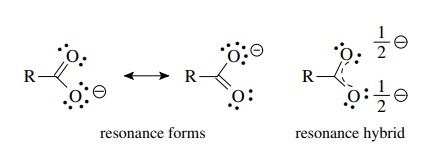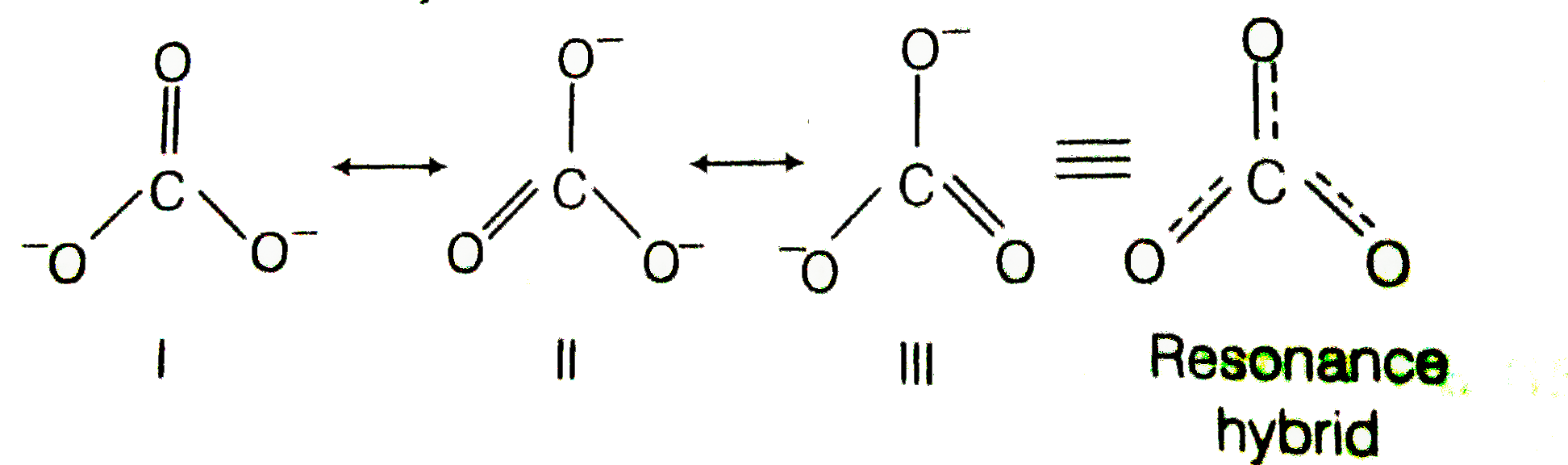
Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

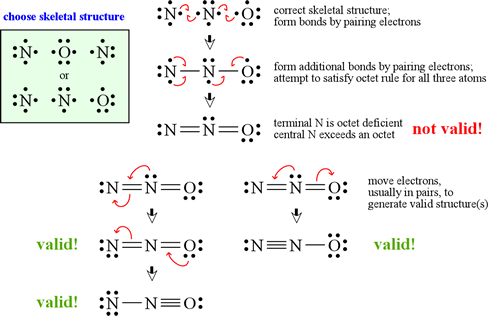
All questions go with Model 4 , please label thank you in the Ltwis suettre for PCls and XeFe Model 4: Which Lewis Structure is Better? The two structures below could represent

Explain why CO_{3}^{2-} ion cannot be represented by a single Lewis structure. How can it be best represented?

Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)